Berlin-Chemie AG
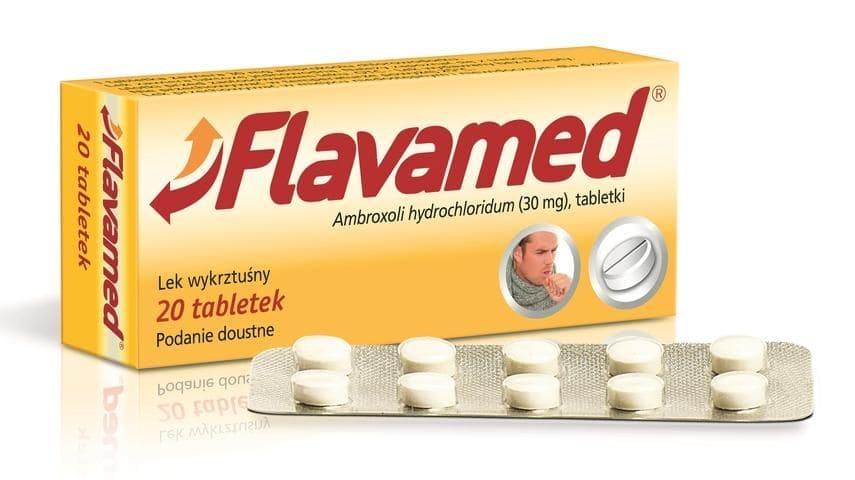
Flavamed Cough Tablets N20 bronchial disease, pulmonary
Flavamed Cough Tablets N20 bronchial disease, pulmonary
Regular price
£38.99 GBP
Regular price
£50.00 GBP
Sale price
£38.99 GBP
Unit price
per
Tax included.
Shipping calculated at checkout.
Couldn't load pickup availability
Flavamed Cough Tablets N20 bronchial disease, pulmonary
Flavamed Cough Tablets N20
PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USERS
Flavamed 30 mg tablets
Flavamed Cough Tablets bronchial disease, pulmonary Ambroxoli hydrochloridum
The leaflet should be read carefully because it contains information important for
the patient.
The drug is available over the counter, to enable the patients to treat some diseases
without consulting a doctor.
However, Flavamed should be used with caution to obtain a good result.
- The leaflet should be kept to be read again if necessary.
- A doctor or a pharmacist should be consulted if advice or additional information is
required.
- If the symptoms aggravate or fail to subside after 4 to 5 days, a doctor should be
consulted.
- If any adverse effect aggravates, or adverse effects not mentioned in the leaflet occur, a
doctor or a pharmacist should be consulted.
Flavamed Cough Tablets bronchial disease, pulmonary:
1. What is Flavamed and what it is used for
2. Information important before Flavamed is taken
3. How Flavamed should be used
4. Potential adverse effects
5. How Flavamed should be stored
6. Other information
Flavamed Cough Tablets N20 bronchial disease, pulmonary:
1. WHAT IS FLAVAMED AND WHAT IT IS USED FOR
Flavamed is a mucolytic agent used in respiratory tract disorders.
Flavamed is used in pulmonary and bronchial diseases associated with production of thick
mucus. Owing to the effect of Flavamed, thick mucus becomes more liquid and easier to
expectorate.
2. INFORMATION IMPORTANT BEFORE FLAVAMED IS TAKEN
Flavamed should not be used in the following cases
- if the patient is found to be allergic (hypersensitive to the active substance of Flavamed
(ambroxol hydrochloride) or any of the other components of the drug, see section 6 „What
Flavamed contains”).
- in children below 6 years of age.
Flavamed should be used with particular caution
- if the patient had in the past severe allergic skin reactions (Stevens-Johnson syndrome,
Lyell syndrome).
• Stevens-Johnson syndrome is a condition characterized by high fever and vesicular skin
eruptions on the skin and mucous membranes.
• life-threatening Lyell syndrom is also known as burnt skin syndrome.
The symptoms include severe skin lesions in the form of vesicles similar to those observed in
scalds and burns.
Flavamed Cough Tablets N20 bronchial disease, pulmonary:
Therefore, if the patient develops any lesions on the skin or mucous membranes, Flavamed
should be discontinued immediately. A physician should be consulted!
- if the patient has impaired renal function or a severe liver disease. In such cases, Flavamed
should be used with particular caution (i.e. longer intervals between the consecutive doses or
lower doses of the drug – a physician should be consulted). In severe renal failure the
metabolites of Flavamed may ccumulate in the organism.
- if the patient suffers from a bronchial condition associated with increased mucus production
(e.g. ciliary immobility syndrome). In such a case, the mucus cannot be removed from the
lungs. Flavamed should be used only under the supervision of a doctor.
- in patients with a history of gastric and/or duodenal ulcers, a physician should be consulted
how to use Flavamed, because mucolytic agents can damage the gastric mucosa. Before
the use of Flavame a doctor must be consulted.
Children
Flavamed can be used only in children above 6 years of age.
Co-administration of Flavamed with other drugs
The doctor should be informed about all current and recent medications, also those available
over the counter.
Flavamed Cough Tablets N20 bronchial disease, pulmonary:
Cough inhibitors
No drugs inhibiting the cough reflex (so-called cough inhibitors) should be used during the
treatment with Flavamed. The cough reflex is important for elimination of liquefied mucus and
its removal from the lungs.
Pregnancy and lactation
Before the use of any drug a physician or a pharmacist should be consulted.
During pregnancy and breastfeeding, Flavamed can be used only if prescribed by a doctor!
There are no available data concerning the use of the drug during pregnancy and
breastfeeding. However, animal tests indicate that the active substance is excreted with milk.
Driving and machine operation
Flavamed exerts no effect, or insignificant effect, on the ability to drive and to operate
mechanical devices.
Important information concerning some components of Flavamed
The drug contains lactose (a saccharide occurring in milk). If the patient has been diagnosed
with intolerance of some saccharides, a doctor should be consulted before the drug is used.
3. HOW FLAVAMED SHOULD BE USED
Flavamed should always be used according to the instructions provided in the patient
information leaflet. In case of any doubts, a physician or a pharmacist should be consulted.
The information provided below is applicable if the doctor recommends no other dosage. The
instructions should be followed because otherwise Flavamed will not act correctly.
Flavamed Cough Tablets N20 bronchial disease, pulmonary.
If not indicated otherwise by the doctor, the usual doses are as follows:
Age Single dose Maximum daily dose
Children aged 6 - 12
years
½ tablet 2 to 3 times daily
(corresponding to 15 mg of
ambroxol hydrochloride 2 – 3
times daily).
1½ tablets
(corresponding to 45 mg
of ambroxol
hydrochloride).
Adults and
adolescents above 12
years of age
During the first 2 – 3 days, 1
tablet 3 times daily
(corresponding to 30 mg of
ambroxol hydrochloride 3 times
daily);
then 1 tablet of the drug twice
daily (corresponding to 30 mg of
ambroxol hydrochloride twice
daily).
3 tablets (corresponding
to 90 mg of ambroxol
hydrochloride).
Note:
In adults the dose can be increased to 2 tablets twice a day.
Administration
Flavamed tablets should preferably be swallowed whole, followed by an appropriate amount
of liquid (e.g. water, tea or fruit juice).
The tablet can be divided into two halves.
Duration of treatment
If no physician has been consulted, Flavamed should not be taken longer than 4 – 5 days.
If after 4 – 5 days the symptoms fail to subside, or even aggravate, a doctor should be
consulted immediately!
In case of an impression that the effect of Flavamed is too potent or too weak, a physician or
a pharmacist should be consulted.
Overdose of Flavamed
No severe poisoning symptoms have been observed. Transient agitation and diarrhea may
occur.
In case of significant overdose, increased salivation may occur, as well as nausea, vomiting
and hypotension. A doctor should be consulted. Usually immediate intervention such as
induction of vomiting and gastric lavage is unnecessary; such management can be
considered in case of acute overdose. Treatment dependent on the observed symptoms of
overdose is recommended.
Missing a dose of Flavamed
In case of a missed dose, or too low dose of the drug taken, the next prescribed dose should
be taken at the usual time. No double dose to compensate for the missed one should be
used.
In case of any doubts associated with dosage of the drug, a physician or a pharmacist should
be consulted.
4. POTENTIAL ADVERSE EFFECTS
Like any other drug, Flavamed may cause adverse effects, although they are not observed in
all patients.
Adverse effects are assessed according to the following frequency criteria:
Very frequent In more than 1 out of 10 treated patients
Frequent
In fewer than 1 out of 10, but more than 1 out of 100 treated
patients
Infrequent
In fewer than 1 out of 100, but more than 1 out of 1 000 treated
patients
Rare.
In fewer than 1 out of 1 000, but more than 1 out of 10 000
treated patients
Very rare
In fewer than 1 out of 10 000 treated patients
Unknown (the frequency cannot be estimated on the basis of
available data).
Adverse effects
Gastrointestinal:
Infrequent: Stomachache, nausea, vomiting
Skin and subcutaneous tissue:
Very rare: Severe dermal reactions, such as Lyell syndrome and Stevens-Johnson syndrome
(see section 2 „When Flavamed should be used with particular caution”)
Systemic disorders and local conditions:
Infrequent: Hypersensitivity reactions such as rash, facial edema, respiratory disturbances,
pruritus, fever
Very rare: Severe allergic reactions (anaphylaxia), including anaphylactic shock
Management.
In case of one or more of the aforementioned adverse effects, treatment with Flavamed
should be discontinued.
If any adverse effect aggravates, or adverse effects not mentioned in the leaflet occur, a
doctor or a pharmacist should be consulted.
5. HOW FLAVAMED SHOULD BE STORED
The drug should be stored out of the reach and sight of children.
Flavamed should not be used after the expiry date indicated on the blister and cardboard box
after the abbreviation EXP. The expiry date is the last day of the given month.
The drug should not be stored at temperatures exceeding 25°C.
The drug should not be disposed of in the sewage system or home waste bins. A pharmacist
should be consulted what to do with unwanted drugs. Such disposal method will help to
protect the environment.
6. OTHER INFORMATION
What Flavamed contains:
The active substance – ambroxol hydrochloride.
One tablet contains 30 mg of ambroxol hydrochloride. Other components of the drug include:
lactose monohydrate, corn starch, cellulose (powder), sodium croscaramellose, povidon K 30,
magnesium stearate.
Appearance of the drug and package content
White, round tablets, flat on both sides, with beveled edges and a division groove on one
side.
Flavamed is available in packages containing 10, 20 and 50 tablets.
Flavamed Cough Tablets bronchial disease, pulmonary
Responsible entity and manufacturer:
Berlin-Chemie AG
Glienicker Weg 125
12489 Berlin, Germany